PRINCETON, N.J. –(ENEWSPF)– June 14, 2017 – Bristol-Myers Squibb Company (NYSE: BMY) is voluntarily recalling one lot (#HN0063) of Eliquis 5 mg tablets to the consumer level. This lot was distributed nationwide in the U.S. to wholesalers and retail pharmacies in February 2017. Bristol-Myers Squibb is taking this precautionary measure based on a customer complaint that a bottle labeled as Eliquis 5 mg was found to contain Eliquis 2.5 mg tablets.
Patients should not stop taking Eliquis without consulting with their physician. Patients who are prescribed Eliquis 5 mg for an irregular heartbeat (atrial fibrillation) and take an Eliquis 2.5 mg tablet instead, particularly for a prolonged period, would have an increased probability of stroke, a moving blood clot, or death. For patients with Deep Vein Thrombosis (DVT), a blood clot in one of the deep veins usually in the leg, and Pulmonary Embolism (PE), a blood clot in the lung, underdosing of the drug could lead to an increased risk of a growing or moving blood clot. Should that occur, it could be life-threatening or reversible depending on the severity and location of the blood clot. To date, there have not been any reports of injuries or illnesses related to this issue.
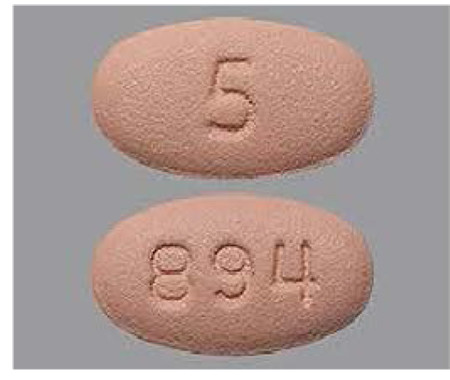
Eliquis tablets are indicated to reduce the risk of stroke and blood clots in people who have atrial fibrillation; it also treats blood clots in the veins of your legs or lungs as well as reduces the risk of forming a blood clot in the legs and lungs of people who have just had hip or knee replacement surgery. Eliquis 5 mg tablets are packaged in 60-count bottles, lot HN0063, Exp 09/2019, NDC 0003-0894-21. The recalled lot was distributed Nationwide in the U.S. to wholesalers and retail pharmacies in February 2017.
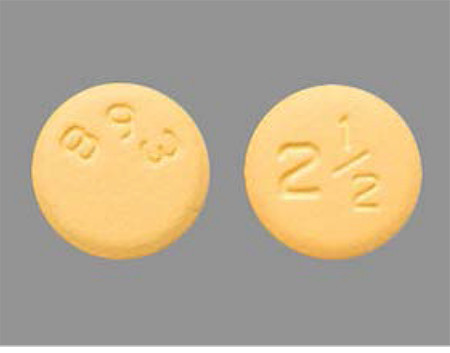
There are distinct visible differences between the two tablet strengths including colors, size and markings that distinguish the 2.5 mg and 5 mg tablets (see photos) and decrease the likelihood of an incorrect dose. The 2.5 mg presentation is a yellow, round, biconvex, film-coated tablet with “893” debossed on one side and “2½” on the other side. The 5 mg presentation is a pink, oval, biconvex, film-coated tablet with “894” debossed on one side and “5” on the other side.
Patient safety is our first priority. Bristol-Myers Squibb has notified wholesalers and pharmacies to arrange for return and replacement of any recalled product. Consumers that have product being recalled (Lot #HN0063) should contact their physician and call the Bristol-Myers Squibb Customer Information Center at 1-800-332-2056, Monday – Friday, from 8 AM – 8 PM EST or visit BMS.com for more information. Please see Eliquis U.S. Full Prescribing Information, including Boxed Warnings .
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration. Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking this drug product.
Complete and submit the report Online: www.fda.gov/medwatch/report.htm. Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
About Bristol-Myers Squibb Bristol-Myers Squibb is a global biopharmaceutical company whose mission is to discover, develop and deliver innovative medicines that help patients prevail over serious diseases. For more information about Bristol-Myers Squibb, visit us at BMS.com or follow us on LinkedIn, Twitter, YouTube and Facebook.
Source: http://fda.gov








